A brief history of ImuRegen
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2019, 88(3), 115-120 ISSN 0372-7025 (Print) ISSN 2571-113X (Online) DOI: 10.31482/mmsl.2019.008
REVIEW ARTICLE
A SHORT HISTORY OF IMUREGEN – AN ORIGINAL TISSUE EXTRACT
Klara Kubelkova and Ales Macela. Department of Molecular Pathology and Biology, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
Received 14th March 2019. Accepted 29th April 2019. Published 6th September 2019.
Summary
Imuregen is a unique dietary supplement that was developed by leading Czech immunologists, made and distributed from the Czech Republic, and first came out in the 1950s. Imuregen boasts of decades of research and clinical exposure. It was created for the immune system sourced only from all natural ingredients that support cellular immunity and energy, tissue regeneration, wound healing, endocrine gland repair, intestinal integrity, memory enhancement, and has antitumor effects.
Introduction
Natural biological products in the forms of teas or extracts were the first drugs for improving the fitness of human beings in ancient times. Over the course of the millennia, an immense amount of empirical experience has been ac- cumulated. As passed from generation to generation, this has given rise to numerous innovative therapies. Their usage continues up to the present day, especially in the forms of prebiotics, probiotics, and/or nutritional supplements to support regeneration or innate immune responsiveness during unspecific sicknesses or during convalescence from infection or cancerous illnesses. The U.S. National Library of Medicine defines the term “tissue extracts” as: “Preparations made from animal tissues or organs (animal structures). They usually contain many components, any one of which may be pharmacologically or physiologically active. Tissue extracts may contain specific, but uncharacterized factors or proteins with specific actions”(1). In terms of their composition, activity, and largely uncharacterized nature, a number of extracts of both plant and animal origin which originated in the middle of the 20th century in former Czechoslovakia fit this description.
Historical aspects
The EKO factory in Prague established an experimental Retisin (RTN) team in the 1940s. It was a small team composed of external and internal specialists collaborating on a project oriented toward the preparation of animal and plant tissue extracts directed against tumors. The results of that team’s experiments were classified as secret and the team members also were bound to secrecy. The founder of the team and its leader was Bohumir Rakusan (1900–1969),
University of Defence, Faculty of Military Health Sciences, Department of Molecular Pathology and Biology, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
[email protected]
+420 973 255 193, +420 973 253 100
 Klara Kubelkova and Ales Macela: History of Imuregen
Klara Kubelkova and Ales Macela: History of Imuregen
Other members were Jaroslav Fanta, Karel Osvald, Gabriel Urbanek, Alois Vystrcil, and Bedrich Dolezel. The RTN team was oriented toward the preparation of tissue extracts that could be classified as what we more recently would term biological response modifiers. Several of the tissue preparations were named and patented (RTN33 – RETISIN (2), RTN112 – LYASTIN (3), RTN121 – SILEXIL (4)).
Retisin was an ethanol extract of bovine tissue. It consisted of 0.5 mg of dry extract per milliliter of double- distilled water. Its developers had declared the active substance to be a color complex and its sodium salt to be water soluble. Retisin was patented on 10 October 1946. The trademark expired on 10 October 2006. Lyastin (RTN112) was an ethanol/ether extract from bovine tissue, and it was declared to be a preparation containing specific phospholipid and proteolipid components. The trademark for registration of the Lyastin preparation was issued on 5 November 1941 and expired on 5 November 2001. Among the other preparations prepared and tested by the RTN team were Silexil, an ethanol extract from milt and roes of herring. Another ether extract, named Sangitin, came from that same source. A preparation known as Floristen (5) was prepared from Hypericum perforatum by ethanol extraction. Additional, unnamed, preparations were RTN119 and RTN 134, prepared by ethanol extraction of bee’s honey. Retisin, Floristen, Lyastin, and other extracts were broadly tested in experiments using various experimentally induced illnesses on experimental animal models (mice, rats, guinea pigs, and rabbits) as well as in clinical practice (6-12). In general, Retisin in experimental animal models has been shown to limit the induction and growth of benzpyrene-induced sarcomas, and the majority of testing teams have shown an anti- inflammatory effect. Clinical testing at the time had revealed positive effects of the tested preparations and that these could be utilized as drugs for supportive therapy. Specifically, Retisin was utilized as a supplement to therapy for oncological illnesses in gynecology, post-irradiation complications in oncology, and as an analgesic (13).
A significant conclusion emerging from the original experiments was that these extracts, namely Retisin and Lyastin, were effective against tumor cells within in vitro cultures and that they were harmless for nontumor cells. Such data may still be interesting for formulating strategy to restrict tumor growth.
The EKO factory came under Communist national administration in 1948, and all movable property and real estates were nationalized and transferred under the state-owned company Biogena. All rights to technical documentation, research, and patents to RTN preparations were transferred to the national pharmaceutical company SPOFA (14). The research on RTN preparations was in fact never properly completed. Some of the preparations, among them Retisin, as the predecessor of the products Juvenil and Imuregen, were put into production on the basis of a political decision in 1958. They were manufactured in injection form until the late 1970s. Finally, Retisin, as well as some other original extracts prepared by the RTN team, was eliminated from production in 1975 under so-called “Action Mars,” which had been intended to modernize the assortment of drugs in the Czechoslovak health care system (15). The decision of the Ministry of Health on Retisin stated that: “the preparation has only a symptomatic effect, it could hide the course of the disease itself – production is stopped” (16).
The worst accident in the history of nuclear energy occurred during a technical test at the Chernobyl nuclear power plant in northern Ukraine on Saturday, 26 April 1986. Responsible persons in the Soviet Union sought ways to protect the affected population from the consequences of radiation exposure. The original Retisin, and the result of its renewed production under the name Juvenil, along with Silexil, were tested for harmlessness and efficacy at several laboratories in the Soviet Union and Poland (17). Subsequently, Retisin, now renamed Juvenil, was tested also in the laboratories of several Czechoslovak institutions. The Center of Nutritional Hygiene declared that: “Juvenil is medically harmless. However, according to the method of ‘rejuvenating tissue therapy’ proposed by prominent Soviet biologist professor Vladimir Filatov, Juvenil does not show extraordinary biological stimulating effects.” All the results from Juvenil testing by the institutions mentioned above were evaluated and interpreted in conformity with Filatov’s theory of so-called ,,external therapy” according to which those substances that are produced in large quantities during decomposition or destruction of tissues have a large stimulatory effect when absorbed through the skin.
Juvenil was tested also at the Techonin Research Laboratory of the Purkyne Military Medical Academy, Hradec Kralove, Czechoslovakia. The military experts, based on the data from original testing, suggested that the Juvenil preparation is a biological response modifier and not merely a nutritional supplement or “rejuvenating” preparation. For this reason, Juvenil was tested according to the published “Multistep scheme for testing immunomodulatory substances”(18). The results were summarized in a research report dated 12 June 1989 (19). According to the results of the test utilized for Juvenil, the preparation has no mitogenic activity when tested in vitro on murine spleen cells. When given in vivo per os to mice (seven consecutive days), Juvenil significantly increases the readiness of lymphoid cells (T and B lymphocytes) for response to mitogen (the test was carried out 24 h after the last administration of Juvenil). In a co stimulatory test, however, Juvenil generally and significantly interfered with the activity of Con A (T-cell mitogen) in the same T-cell subtypes. The co stimulation with PHA (another T-cell mitogen) and LPS (a B-cell mitogen) was rather negligible, if any occurred at all. Juvenil was tested also for its potential ability to initiate the activity of cytotoxic cell populations, specifically, the activity of natural killer cells (NK cells) and macrophages. The results of both tests revealed the ability of Juvenil to induce NK cell activity against NK cell-sensitive cellular targets as well as to induce cytotoxicity of murine peritoneal macrophages against two different standard target cell lines. The preliminary results also demonstrated that Juvenil administered in vivo increased resistance to mild pathogenic bacteria, but its effects against fully virulent bacteria were negligible. Based on the initial results, a change was recommended in Juvenil’s administration form from that of liquid drops to pills. The pills, under the name Stimunal, were tested against infection with two strains of the intracellular bacteria Francisella tularensis. The preliminary results revealed protective effect only against the strain with very low virulence [20].
In parallel with examining the immunomodulatory activity of Juvenil, and in accordance with the general task originally assigned to evaluate induction of resistance (protection) against γ irradiation (Co60), the material was tested on mice. These experiments were done at the Department of Radiobiology, Purkyne Military Medical Academy, Hradec Kralove, Czech Republic. This series of experiments was never finalized due to a reorganization of the Academy. From preliminary evaluation of radioprotective effect, however, it can be said that Juvenil probably influences the redistribution of stem cells among bone marrow, spleen, and periphery. Thus, important information about several basic characteristics in functional profile for the ethanol or ethanol/ether extracts of bovine tissue were accumulated up to the late 1980s.
The last decade of the 20th century brought essentially a rebirth for this interesting preparation. This was due to an increased interest within Central Europe in alternative medicine and natural products, including in natural dietary supplements that might eliminate the impacts of technological and civilization stressors on the health of the human population. The company Uniregen, Ltd. in Nachod, Czech Republic, prepared a new batch of the bovine tissue extracts under the original formula, renamed it Imuregen, and invited several expert groups from different institutes to participate in reevaluating the biological effects of Imuregen.
The first information from this era can be obtained from the final research report of the project “Protective and immunomodulative influence of supplementing DNA: comparing of clinical testing and experimental model,” financed by the Internal Grant Agency of the Ministry of Health of the Czech Republic (21). The Health Institute, Usti nad Labem; Microbiological Institute of the Czech Academy of Sciences, Prague; Medical Faculty of Charles University, Prague; and Institute of Anatomy, Medical Faculty of Charles University, Hradec Kralove (all in the Czech Republic) participated in the aforementioned project. Immediately when new testing began, a discussion began on how to interpret the results of the model studies. There were only two working hypotheses considered in evalu- ating the results of the studies. These related to (1) the effects of nutritional supplementation due to the nucleotides and other low-molecular weight components within the extract, and (2) as a result of the already mentioned modulation of biological responses, mainly the question of whether the material participates in the innate immune responsiveness. There may in fact be no essential difference between these two views on molecular interactions, because both are causing functional changes in the evaluated cell systems.
The results of the aforementioned research project demonstrated in a murine model that a 30-day preventive application of Imuregen in the form of a drinking regime substantially increased levels of regulatory cytokines, and especially of those which are important for the development of antitumor immunity. In parallel, the work revealed stimulatory effects on the differentiation and activity of cytotoxic T lymphocytes and NK cells. The same experimental setup applied to analyzing the effect of Imuregen on antibody-producing cells showed increase in the absolute number of cells producing IgM and IgG antibodies in the spleens of treated animals. In all experimental arrangements used, the condition of experimental animals was monitored. From the data thus obtained, it was concluded that for mice the 30-day drinking regime of Imuregen showed no evidence of toxic effects. Histological examination of gut epithelium proved a positive influence of Imuregen on regeneration and growth of terminal ileum epithelium.
Field experiments focused on preventive application of Imuregen as a nutritional supplement were conducted in three blocks: supplementation of preschool children, school children, and senior population. In all three blocks there was demonstrated significant reduction in sickness, decreased morbidity, and significantly fewer sick days (21). During clinical testing, nutritional supplementation with Imuregen demonstrated significant change in laboratory parameters and improvement in clinical indications of symptoms among patients with chronic fatigue syndrome. In children with allergies, Imuregen improved the clinical and laboratory parameters. It should be noted that, in this case, there had been an interaction of two independent treatment methods, including a combination of speleotherapy and Imuregen supplementation. Noteworthy is that the effect of Imuregen supplementation was superior to that of speleotherapy itself. The results of the project also proved that supplementation with Imuregen contributed substantially to reparation of immune functions in sick persons affected by ulcerative colitis. The majority of the project’s results were published in scientific journals with strict peer review policies (22-24).
Based upon the results obtained thus far, Imuregen is recently being combined with other biological response modifiers, such as Pleuran, which is an agonist in the trained immunity, and/or other natural, biological products (biological fibers, Ginkgo biloba, minerals or vitamins) that can constitute effective nutritional, metabolic, or cognitive enhancers. All the information on the functional profile of Imuregen presented in this short history has demonstrated the versatility of Imuregen’s actions, which, moreover, reflect the definition of a tissue extract.
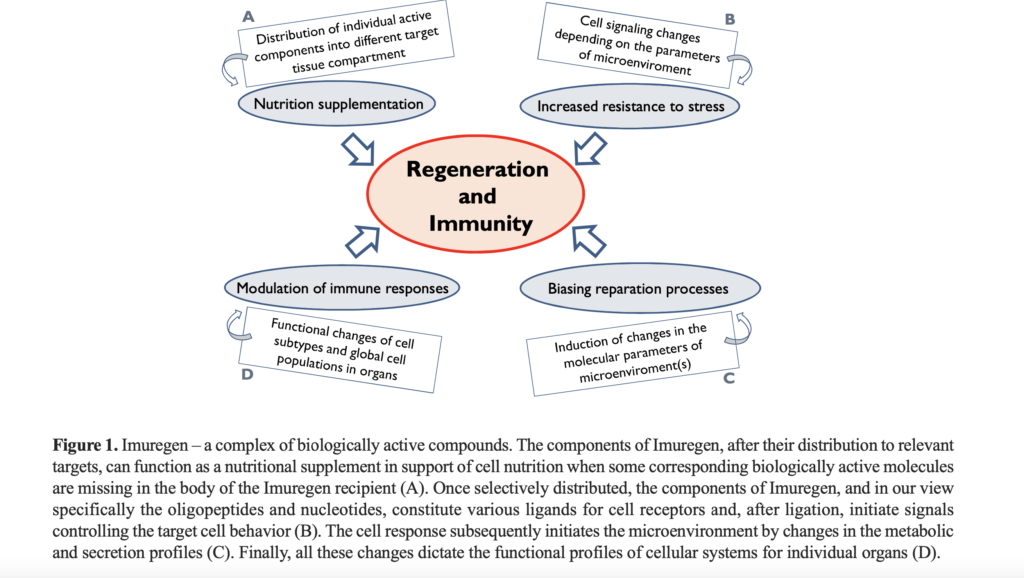
As a complex of biologically active low molecular weight components, Imuregen constitutes a versatile modulator of biological responses. In accordance with recent information on the broad spectrum of Imuregen’s biological activities, it can be said in summary that individual active Imuregen components seem to be distributed into different tissue compartments of the body where they interact directly with the cells there. The direct interaction of the components can be facilitated solely by such of their basic molecular characteristics as molecular weight, charge, and/or primary sequence of component construction units (amino acids and/or nucleotides). These can be ligands for cell surface receptors or signals for so-called damage-associated molecular pattern receptors. Subsequent to interaction, individual components may induce changes in activation of cellular signaling pathways that influence the functional profile of responding cells and, in parallel, influence the intercellular signaling that controls the tissue microenvironment and, directly, the parameters of response to stressors (Fig. 1) see below.
Conclusion
Such is the short history of one of the original preparations emerging in the Czechoslovakia. Nontoxic and apparently free of side effects, Imuregen and Imuregen-derived products seem to be promising tools for mitigating various stressor influences such as infections, different types of irradiation, some immunological disorders, and nonlethal deviations in the metabolism. Due to the broad spectrum of the tissue extract’s modulatory activity, however, such preparations must be utilized only to a reasonable extent and, in cases of disease, consultation with one’s doctor or pharmacist would be advisable. Meanwhile, a product demonstrating such promising broad modulation of biological responses deserves further targeted research to uncover the molecular basis of its effects.
Author contributions
All authors contributed equally to the work.
Funding
This work was conducted within the framework of Ministry of Defence of the Czech Republic – long-term organization development plan Medical Aspects of Weapons of Mass Destruction of the Faculty of Military Health Sciences, University of Defence.
Conflict of Interest
The authors declare that they have no conflicts of interest regarding the publication of this article.
Adherence to Ethical Standards
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors.
References
- 1. https://www.urmc.rochester.edu/profiles/display/124207
- 2. https://isdv.upv.cz/webapp/ozs.det?pozk=1305&plan=cs&s_naze=RETISIN&s_sezn=%2520&s_
- 3. https://oz.kurzy.cz/spofa-as/lyastin-p4163z91087u.htm
- 4. https://oz.kurzy.cz/spofa-as/silexil-p4159z101165u.htm
- 5. https://oz.kurzy.cz/spofa-as/floristen-p2114z151529u.htm
- 6. Urbanek G, Dolezal B, Rakusan B, Vystrcil A, Zicha K, Zicha O. [Treatment of endarteritis with tissue preparations RTN]. Cas Lek Cesk. 1952 Nov 14;91(45-46):1375-7.
- 7. Dolezal B, Rakusan B, Urbanek G, Vystrcil A, Zicha K, Zicha O. [Retisin, a new tissue preparation]. Cesk Farm. 1954 Sep;3(7):246-7.
- 8. Dolezal B, Rakusan B, Urbanek G, Vystrcil A, Zicha K, Zicha O. [Floristen as a drug in the treatment of inflammation]. Cesk Farm. 1954 Sep;3(7):247-8.
- 9. Urbanek G, Dolezel B, Rakusan B, Vystrcil A, Zicha K, Zicha O, Lundova A. [Therapy of chronic gynecological diseases with the tissue preparation Floristen mite (RTN 118)]. Cas Lek Cesk. 1954 Dec 3;93(49):1352-4.
- Dolezal B, Rakusan B, Urbanek G, Vystrcil A, Zicha K, Zicha O. [Floristen as a drug in the treatment of inflammation]. Cesk Farm. 1954 Sep;3(7):247-8.
- Kolarova O. [Comparison of therapeutic results in inflammations of the adnexa obtained with Trypsin, Adnexbacterin, Floristen and Betalactin (author’s transl)]. Cesk Gynekol. 1973 Sep;38(8):589-90.
12.Pavlovsky P, Hanakova B, Petranova V. [The use of Floristen and Lyastin in arteriosclerotic affectionof the brain]. Cesk Psychiatr. 1979 Apr;75(2):118-21.
13. Dolezal B., Rakusan B., Urbanek G., Vystrcil A., Zicha, K., Zicha O.: [Existing experiences with tissuepreparations RTN]. Military Medical Letters, 1956 Suppl. 1, Part II.: 3-14 (Czech).
- Decree of 27 June 1948 on the nationalization of enterprises pursuant to Act No. 114/1948 Coll. of 27.6.1948, Collection: 1256/1948 U.l.i, Amount: 126/1948.
- Jakoubkova J.: [Tissue preparation Retisin – the drug for patients with tumors?]. Prakt. Lek., (Praha), 59, 1979 (23):894.
- The letter send from Ministry of Health of Czechoslovak socialist republic, dated February 24, 1978.
- The research report [“Evaluation of biological effects of Retisin, Silexil, and Juvenil on tissue cultures of kidney cells – the summary of results”]. Dated July 13, 1986 (Russian).
18.Macela A, Kovarová H, Stulik J, Ferencik M, Propper P, Hoskova E. Multistep scheme for testing immunomodulatory substances. Bratisl Lek Listy. 1989 Oct;90(10):719-31 (Czech, English).
- The research report [The results of preliminary Juvenil testing on immunomodulatory activity]. Dated June 12, 1989 (Czech).
- The report on experiment [Stimunal] Dated December 17, 1990 (Czech).
- Richter J, Sima P, Pfeifer I, Kral V, Jilek D, Stiborova I, Richterova S, Peskova J, Slizova D, Dobiasova L. Protective and immunomodulative influence of supplementing DNA: comparing of clinical testing and experimental model. Research report, January 29, 2004:1-61.
- Slizova D, Sima P, Richter J, Krs O, Zavadilova J. Stimulation of ileal epithelium growth and regeneration by dietary nucleotide extracts. Acta Medica (Hradec Kralove), 2004;47(3):163-166.
- Richter J, Sima P, Pfeifer I, Turek B. Nucleotides and their role in health and disease. Ceske pracovni lekarstvi,2004;5(1):27-32.
- Richter J, Svozil V, Kral V, Rajnohova-Dobiasova L, Stiborova I, Pohorska J, Vetvicka V. Effects of dietary nucleotides on immune mechanisms and physical state in children with chronic respiratory problems. Am. J. Immunol. 2015;11(2):26-32.
See original article here. https://www.mmsl.cz/pdfs/mms/2019/03/03.pdf

Worldwide Shipping
We offer fast and reliable shipping options to get your Imuregen wellness essentials to your doorstep in no time.

Best Quality
Your health is our top priority, and we are committed to providing you with the highest quality products

Best Offers
We're confident in the quality of our products, and we want you to be too. That's why we offer a satisfaction guarantee on all Imuregen purchases.

Secure Payments
Imuregen offers a variety of secure payment options to make your shopping experience hassle-free.